- Abteilungen
- Abteilung Symbiose
- Forschungsgebiet
- Symbiose in ...
Symbiose in ...
Gutless oligochaetes
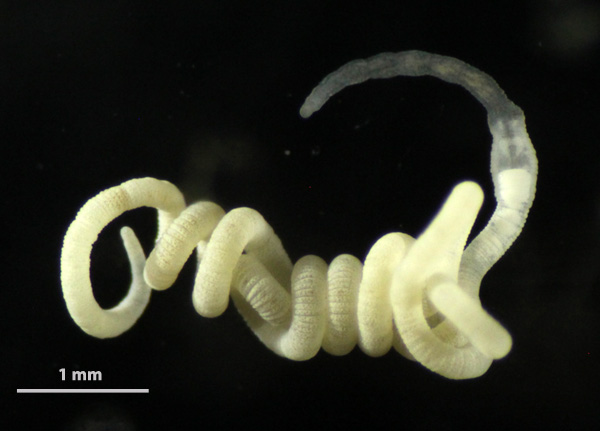
Gutless oligochaetes are a unique group of marine worms that lack a mouth, gut and anus. They have also lost their excretory organs (nephridia) and rely on chemosynthetic bacterial symbionts for both nutrition and waste disposal. Gutless oligochaetes are typical meiofauna organisms that inhabit marine sand and mud bottoms, mostly in subtropical and tropical regions. Worldwide around one hundred species have been scientifically described.
We use microscopic, molecular, analytical and experimental approaches to understand the biology, evolution and diversity of these symbiotic systems.
In contrast to intracellular endosymbionts that are not directly exposed to the external environment of the host, the endosymbionts of gutless oligochaetes are extracellular, living just below the "skin" of the worms in a space between the outer cuticle and the internal epidermal cells called the symbiont-containing region. The cuticle is highly permeable to both small charged molecules and uncharged substances so that the symbiotic bacteria have free access to most dissolved compounds in the sediment pore waters surrounding the worms.
Our studies on the phylogeny of the oligochaete bacteria have revealed a remarkable diversity of the symbionts, with up to 5 - 6 different 16S rRNA phylotypes co-occurring in the symbiont-containing region. In all host species, the primary endosymbionts are chemoautotrophic sulfide-oxidizers that belong to the gamma subclass of Proteobacteria and are related to free-living sulfide-oxidizers such as Allochromatium vinosum. In addition to the primary symbionts, up to 5 other bacterial phylotypes belonging to the Alphaproteobacteria (Blazejak et al. 2006), Deltaproteobacteria, or the Spirochaetes can co-exist in the same host species (Dubilier et al. 2006).
Phylogenetic analyses of the oligochaete symbionts and their hosts suggest that both co-speciation and biogeography played a role in the establishment of these symbioses. Different transmission modes of the symbionts may influence their evolutionary patterns.
For the oligochaete hosts, the association is clearly obligate, given their complete reduction of a digestive and excretory system. For the symbionts, that remain as yet uncultivable, it is not known if they can also survive in a free-living stage in the environment.
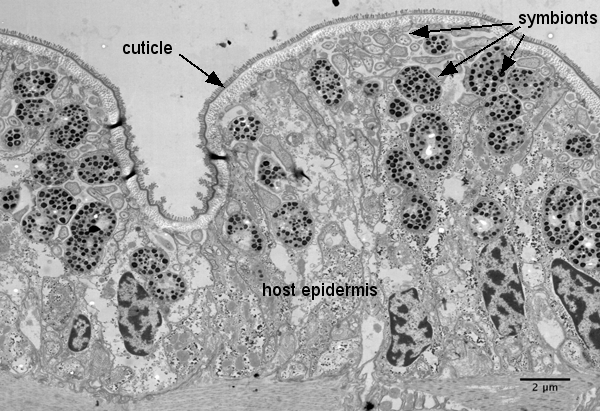
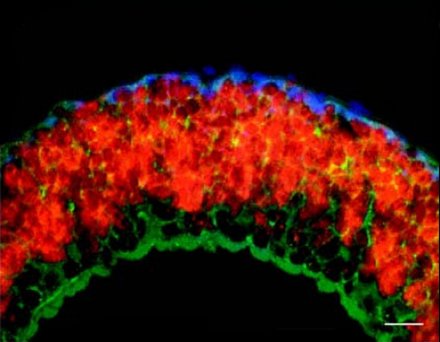

It is, however, clear that the symbionts benefit from the worms. Marine sediments are stratified with oxygen being present in the upper and reduced compounds in deeper layers. The worms can actively alternate between two layers thereby providing the symbionts with all the compounds they need.
One of our main study sites is on the island of Elba (Italy). Here, gutless oligochaetes occur abundantly in the vicinity of Posidonia oceanica sea grass meadows. Decomposing sea grass rhizome releases inorganic substrates that can be used by the chemosynthetic bacteria.
Current projects
- Transmission of symbionts (Alexander Gruhl, Mario Schimak, Juliane Wippler)
- Storage compounds and symbiont uptake(Juliane Wippler, Alexander Gruhl)
- Speciation and host-symbiont coupling (Yui Sato)
- Comparative and functional genomics of deltaproteobacterial symbionts and their free-living next relatives (Anna Mankowski, Juliane Wippler)
- Symbiont replacement in gutless oligochaetes (Anna Mankowski, Juliane Wippler)
- Symbiont community metagenomics (Juliane Wippler, Anna Mankowski)
- The unusual sterols profile of Olavius algarvensis
(Dolma Michellod) - Host morphology and development (Alexander Gruhl)
- The role of respiratory pigments in the Olavius holobiont (Sidney Fulford, Pierre Methou, Alexander Gruhl)
Stilbonematid nematodes
Judith Zimmermann, Nikolaus Leisch
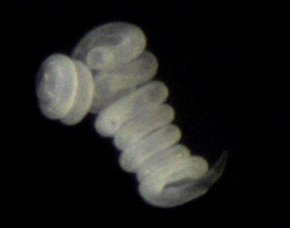
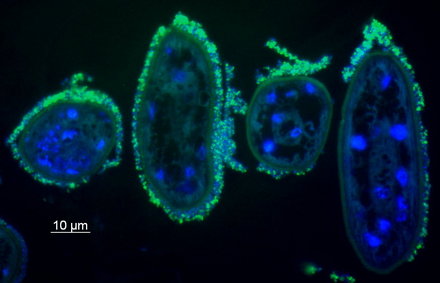
Our understanding of the function of stilbonematinid symbioses is still extremely limited. For example, the role of the bacteria is still unclear. One hypothesis postulates that the host benefits nutritionally from the ectosymbionts by grazing on its own coat. We are currently testing this hypothesis with incubation experiments and NanoSIMS. The bacteria may benefit from the mobility of the host through the oxic and anoxic sediment layers, as this would provide them with a regular supply of the reduced and oxidized compounds it needs for its nutrition.
In our group we investigate the following topics:
- Diversity and specificity of the stilbonematid ectosymbioses
- Evolutionary history of the symbioses
- Benefits of the symbiosis for both partners
In this project we work closely together with Joerg Ott, University of Vienna, Austria.
Marine flatworms
Paracatenula is a genus of small marine flatworms that live in the pore water of sands in warm temperate and tropical regions all around the globe. The worms have no mouth or gut and instead rely on intracellular chemosynthetic endosymbionts for nutrition. The bacteria are aerobe sulfur oxidizers, that need both oxygen and sulfide to generate the energy for carbon fixation and the rest of their metabolism. By migrating through the upper layers of the sand, the worm is the shuttle between the oxygenated layers in the top and the sulfidic layers 10–15 cm deep in the sand. The bacterial symbionts can make up to half of the total biomass of the worm and are housed in bacteriocytes that form the trophosome, the nutritive tissue.
The Paracatenula symbionts are so far the only known chemoautotrophic symbionts of the order Alphaproteobacteria. All other known chemoautotrophic and sulfur-oxidizing symbionts are either Gamma- or Epsilonproteobacteria. Their symbionts, named Candidatus Riegeria, have been vertically transmitted for over 500 million years ago. Such a long period of co-evolution suggests that Ca. Riegeria symbionts would show severe signatures of genome reduction and would only have a very limited set of metabolic functions. Just recently we could show that the symbiont of a mediterranean Paracatenula species has an unexpectedly versatile energy and storage metabolism despite its reduced genome. The Ca. Riegeria symbionts can fix carbon, store carbon in multiple soluble and non-soluble compounds and produce all necessary building blocks for cellular life, be it sugars, nucleotides, amino acids or vitamins. In contrast to other chemosynthetic symbioses, the Paracatenula host does not digest its symbionts for nutrient acquisition. Instead, the symbionts feed their host with outer-membrane vesicles (OMVs) that the symbionts secrete. With their high storage capabilities and the elegant ‘current’ like nutrition via OMVs, the symbionts form a bacterial battery for the highly integrated holobiont.


Ongoing projects
- Comparative genomics of the Ca. Riegeria thiotrophic endosymbionts
- Host evolution – the tale of the marine catenulids
- Long-term cultivation – the quest to get chemosynthetic worms to reproduce in the lab

Recent press
Chemosynthetic symbiont with a drastically reduced genome serves as primary energy storage in the marine flatworm Paracatenula (2019). O. Jäckle, B. K. B. Seah, M. Tietjen, N. Leisch, M. Liebeke, M. Kleiner, J. S. Berg, H. R. Gruber-Vodicka. In PNAS.
https://doi.org/10.1073/pnas.1818995116
“Gutless worms rely on handouts from internal microbes” in Nature research highlights.
https://www.nature.com/articles/d41586-019-01147-0
TWIM podcast on the PNAS Paracatenula paper ('the microbial liver')
http://www.microbe.tv/twim/twim-197/
Mouthless in marine sediments – symbiosis makes it possible (2018). O. Jäckle. In: Hempel G, Hempel I, Hornidge A-K, editors. Scientific Partnership for a Better Future. Bremen, Germany: Edition Falkenberg. pp. 25–28.

Recent press
Chemosynthetic symbiont with a drastically reduced genome serves as primary energy storage in the marine flatworm Paracatenula (2019). O. Jäckle, B. K. B. Seah, M. Tietjen, N. Leisch, M. Liebeke, M. Kleiner, J. S. Berg, H. R. Gruber-Vodicka. In PNAS. https://doi.org/10.1073/pnas.1818995116
“Gutless worms rely on handouts from internal microbes” in Nature research highlights. https://www.nature.com/articles/d41586-019-01147-0
TWIM podcast on the PNAS Paracatenula paper ('the microbial liver') http://www.microbe.tv/twim/twim-197/
Mouthless in marine sediments – symbiosis makes it possible (2018). O. Jäckle. In: Hempel G, Hempel I, Hornidge A-K, editors. Scientific Partnership for a Better Future. Bremen, Germany: Edition Falkenberg. pp. 25–28.
Ciliates
The ciliate Kentrophoros has sulfide-oxidizing ectosymbionts, and lives in shallow marine sediments. Like the symbiont-bearing nematodes and gutless oligochaetes, which are also found in the interstitial habitat, they are able to migrate within the sediment to follow gradients of oxygen and sulfide. Despite being single cells, individuals of Kentrophoros can range from a tenth to several millimeters in length.
These curious organisms have been known to biologists for almost a century, but the phylogenetic identity of the bacterial ectosymbionts are still unknown. Using methods of molecular ecology and phylogenetics, we are investigating the identities of both hosts and symbionts. Our preliminary results show that the ectosymbionts belong to the Gammaproteobacteria but are separate from the clade containing nematode and oligochaete symbionts. We are also interested in testing whether the symbionts are host-specific and co-diversify with their hosts.