Project Leader
- Departments
- Department of Molecular Ecology
- Seeps and Vents
- Hydrothermal vent ecology
Hydrothermal vent ecology
Project Leader
Department of Molecular Ecology
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2202 |
|
Phone: |

Since 2005 free-living microbial communities at hydrothermal vents are investigated by members of the Department of Molecular Ecology. Currently our research is conducted within the framework of the Cluster of Excellence “The Ocean Floor – Earth’s Uncharted Interface” lead by MARUM (University of Bremen), and in close collaboration with scientist from different disciplines (e.g. geologists, oceanographers, fluid chemists). The aim of our highly interdisciplinary studies is an advancement of our knowledge of geosphere - biosphere interactions at hydrothermal vents. This includes the purely exploratory description of microbial communities at newly discovered hydrothermal vents. Our main focus, however, is the identification and characterization of microbial key players along physical and geochemical gradients encountered at hydrothermal vents, from areas of hydrothermal discharge to areas where venting has ceased and in the far-field, in order to understand their function within the system and their impact on element cycling.
Field work/Study areas
Study areas are located along the Mid Atlantic Ridge between Azores and Equator (Menez Gwen, Lucky Strike, Rainbow, Broken Spur, Logatchev, Semenov, Ashadze), and in the South Pacific (Manus Basin, Kermadec Arc, Tonga Arc) and Indian Ocean (Kairei).
Research topics
Our previous work includes, e.g., bacterial sulphur cycling in hydrothermal sediments (Schauer et al., 2011), niche partitioning of sulphur oxidising bacteria along the mixing gradient (Meier et al., 2017, Dede et al. 2022), the characterization of heterotrophic microbial communities in the vicinity of hydrothermal discharge (Meier et al, 2016) and in hydrothermal plume (Dede et al. 2023), as well as structure and function of microbial communities inhabiting active and inactive hydrothermal chimneys (Meier et al., 2019; Pjevac et al., 2018).
Our current research focus lies on the effect of trace metals, emitted from the vents, on the ecology of microorgansims and conversely, the effect of microrganisms on the stabilization of trace metals, a prerequisite for an effect of these metals on microbial communities in the far-field.
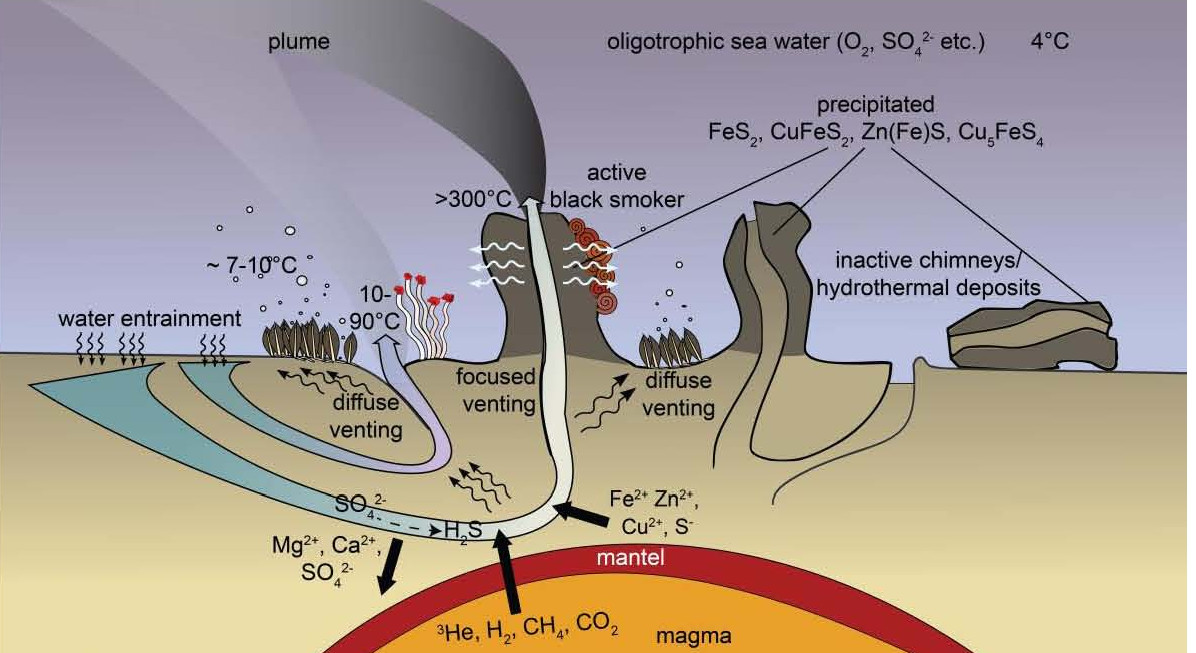
What are deep-sea hydrothermal vent systems?
Deep-sea hydrothermal vents are unique habitats for life. They occur, e.g., along mid-ocean ridges, in bac-arc basins, and at submerged island arc volcanoes in areas where a heat source such as a magma chamber is located close to the seafloor. At these sites, cold oxygenated seawater entrained in the rock gets heated and chemicaly modified before it is again emitted into the water column as high temperature (generally >200°C) fluid at focused and low temperature (generally <100°C) fluid at diffuse vents. These fluids are, depending on the geological settings, enrichted in reduced compounds such as hydrogen sulphide, hydrogen, methane and iron. They are used by chemoautotrophic microorganisms, building up biomass which can further be used as energy source by heterotrophic organisms altogether leading to unique, highly productive oases amidst the predominantly food-limited deep-sea.
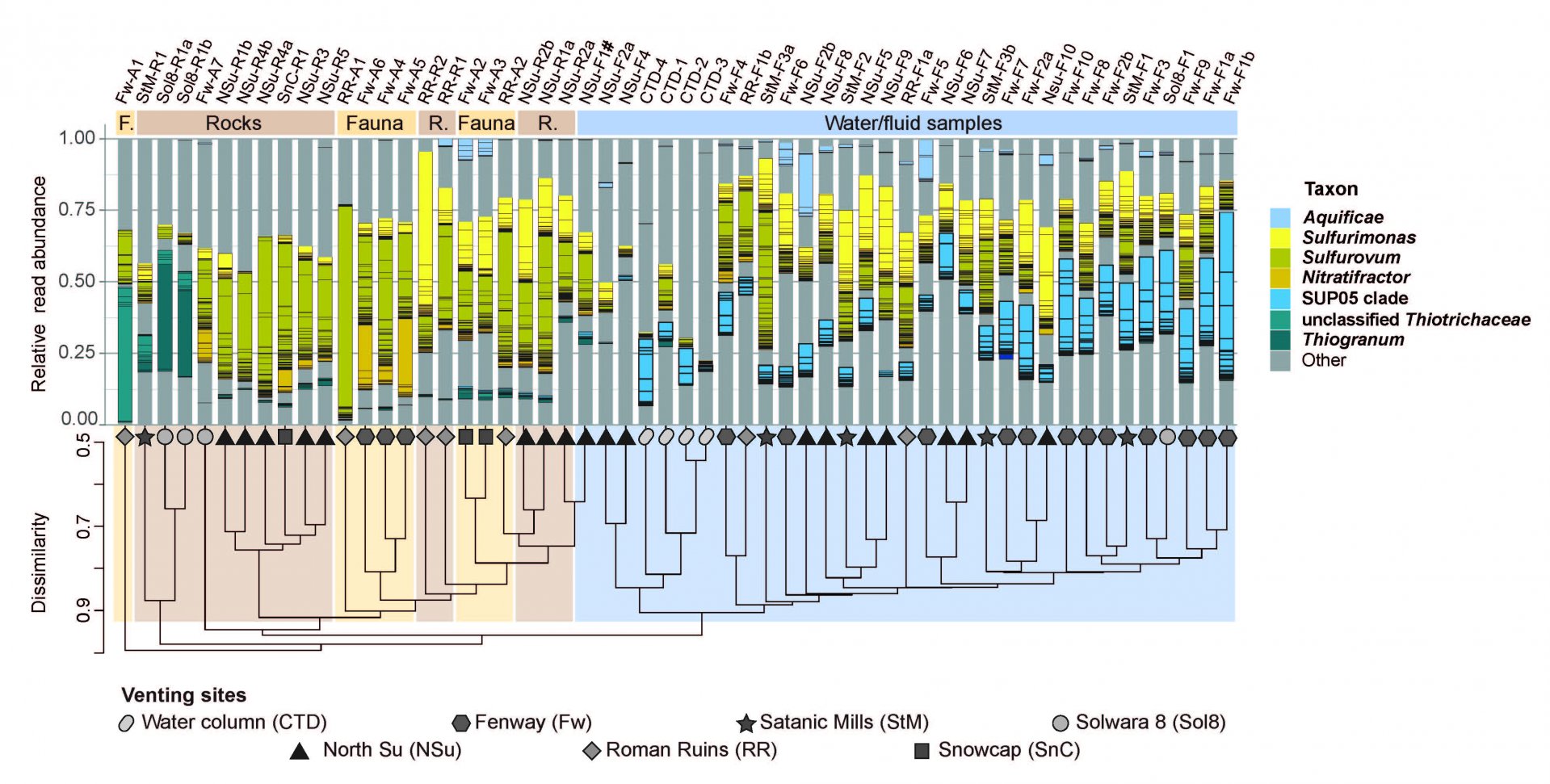
Niche-partitioning of co-occurring sulphur-oxidising bacteria in the Manus back-arc basin
Primary production at deep sea hydrothermal vents, is carried out by chemolithoautotrophic microorganisms, with the oxidation of reduced sulfur compounds being a major driver for microbial carbon fixation. As a systematic study investigating niche-partitioning of co-occurring SOB at hydrothermal vents was still missing, and as a follow up of Pjevac et al. (2014), we systematically investigated niche differentiation of key sulfur-oxidising bacteria in the Manus back-arc basin (Meier et al., 2017). Thirtythree diffuse fluid and water column samples and 23 samples from surfaces of chimneys, rocks and fauna were subjected to a combined analyses of 16S RNA gene sequences, metagenomes and real-time in situ measured geochemical parameters. We found Sulfurovum-related Campylobacterota mainly attached to surfaces exposed to diffuse venting, while SUP05-clade Gammaproteobacteria dominated the bacterioplankton in highly diluted mixtures of vent fluids and seawater. The high diversity observed for Sulfurimonas- and Sulfurovum-related Campylobacterota was proposed to be caused by the high variation of environmental parameters such as oxygen and sulfide concentrations across small spatial and temporal scales.
Niche differentiation of SUP05-clade Gammaproteobacteria in highly diluted mixtures of vent fluids and seawater was further investigated by Dede et al. (2022). In two bathy- and a mesopelagic plumes on the Kermadec intra-oceanic arc in the South Pacific Ocean SUP05 abundance ranged from 22% to 51% relative abundance. In each of the three plumes analyzed, the community was dominated by a different yet uncultivated chemoautotrophic SUP05 species, provisionally named, Candidatus Thioglobus vadi (McV), Candidatus Thioglobus vulcanius (BrV-cone) and Candidatus Thioglobus plumae (BrV-NWC). Statistical analyses, genomic potential and mRNA expression profiles suggested a SUP05 niche partitioning based on sulfide and iron concentration as well as water depth. A fourth SUP05 species was present at low frequency throughout investigated plume samples and may be capable of heterotrophic or mixotrophic growth. We propose that small variations in environmental parameters and depth drive SUP05 niche partitioning in hydrothermal plumes.
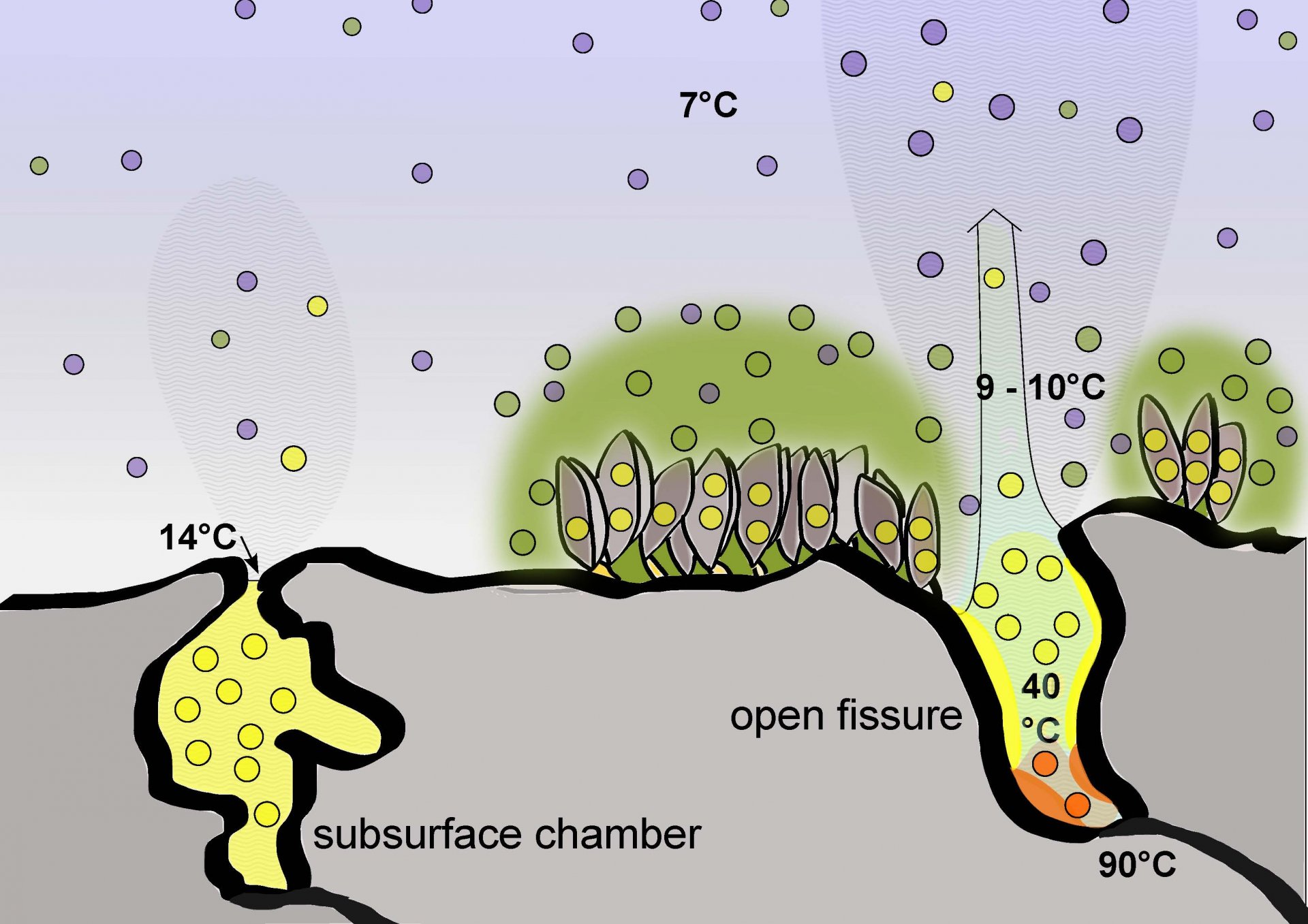
Heterotrophic bacteria at deep-sea hydrothermal vents
Heterotrophic bacteria play an important role in re-mineralisation of carbon fixed by chemolithoautotrophs, closing the carbon cycle in vent ecosystems. Nevertheless, before the background of the unique chemosynthetic capabilities of these environments, heterotrophic microorgansims are generally understudied.
In three of our studies we targeted these yet under investigated heterotrophic part of free-living microbial communities at vents. We could find various Gammaproteobacteria assimilating acetate in diffuse fluids from the Manus Basin bac-arc basin, and identified a novel clade of Nautiliales-related Campylobacterota in diffuse fluids at the Menez Gwen hydrothermal field on the Mid Atlantic Ridge (Winkel et al., 2014). At Menez Gwen we also studied mixing gradients of hydrothermal fluids with seawater from the diffuse fluid discharge points on, up to the buoyant parts of hydrothermal plumes, applying a combination of high throughput 16S rRNA gene tag sequencing, fluorescence in situ hybridisation, and targeted metagenome analysis, combined with geochemical analyses and thermodynamic considerations (Meier et al. 2016). Close to the discharge areas of diffuse fluids, which were dominated by chemolithoautotrophic Campylobacterota, in areas where environmental conditions still supported chemolithoautotrophic processes, we detected microbial communities enriched for versatile heterotrophic Alpha- and Gammaproteobacteria. Analysing genomic bins, the potential for alkane degradation could be shown for several genera and yet uncultured clades. The results suggests that diffuse fluids already contain to a low extend microorganisms that can quickly respond to the availability of carbon compounds, and that these hotspots of chemolithoautotrophic life at hydrothermal discharge points support a “belt” of heterotrophic bacteria significantly different from the dominating oligotrophic microbiota of the deep sea.
In the third study (Dede et al. 2023), we analyzed the microbial communities inhabiting hydrothermal plumes of Niua, Niuatahi and Maka in the Tonga arc (South Pacific). Fluorescence in situ hybridization revealed that Alcanivorax dominated the community at two sites reaching up to 48% relative abundance. We identified that this pattern was driven by two Alcanivorax species in the plumes of Niuatahi and Maka. Despite no indication for hydrocarbon presence in the plumes of these areas, a high expression of genes involved in hydrocarbon-degradation was observed. We hypothesize that the high abundance and gene expression of Alcanivorax is likely due to yet undiscovered hydrocarbon seepage from the seafloor, potentially resulting from recent volcanic activity in this active area. Chain-length and complexity of hydrocarbons, and water depth could be driving niche partitioning in Alcanivorax.
Microbial life on hydrothermal chimneys
Hydrothermal (sulphide) chimneys are the most impressive signboard of deep-sea hydrothermal vents. They form, when hot, acidic, metal- and sulphide-rich fluids, poor in magnesium and sulphate, are emitted into cold oxygenated seawater. Thereby mineral composition and structure of the chimneys strongly depends on the geological setting influencing physico-chemical characteristics of the fluids. Correlating with the steep physico-chemical gradients in such chimney structures, they are inhabited by diverse microbial taxa.
Life on active chimneys
Within the scope of our studies, we found surface scrapes of hydrothermal chimneys recovered from the Manus Basin back-arc spreading system to contain various chemolithoautotrophic Epsilonbacteraeota, while the abundance and distribution of other putative primary producers (e.g., Gammaproteobacteria, Zetaproteobacteria, and Aquificae) was variable between structures of different venting regimes (Reeves et al., 2014; Meier et al., 2017). Using proteogenomics (Pjevac et al. 2018), we identified overlaps in the in situ functional profiles, despite differences in microbial community composition and venting regime. The highly diverse microbial community colonizing a black smoker chimney had a highly redundant metabolic potential. In contrast, the considerably less diverse community colonizing a diffusely venting chimney displayed a higher metabolic versatility. An increased diversity on the phylogenetic level was thus not directly linked to an increased metabolic diversity in microbial communities that colonize hydrothermal chimneys. We could also show that a diversified copy of a key gene of the sox sulfur oxidation pathway, SoxY (SoxY-II), was truly expressed, suggesting a significant role in sulfur cycling at hydrothermal vents.
Life on dead chimneys
Compared to active chimneys, even less is known about the composition and metabolic activity of microbial communities inhabiting inactive massive sulfide chimneys. In another proteogenomic study, we identified two major types of microbial communities, distinctly different from those described for active chimneys, on inactive chimneys of comparable mineralogical origin: One type was characterized by a dominance of Nitrospirae-related putative anaerobic, autotrophic sulfate reducers and the other one by aerobic sulfide-oxidizing autotrophic Gammaproteobacteria (Meier et al. 2019). We provided new insights into the lifestyle of as yet poorly characterized clades, such as Nitrospirae and Gammaproteobacteria of the Woeseiacea-family and the Siboglinidae symbionts-related (SSr)-clade. Our results support the role of the Woeseiaceae-family and the SSr-clade as important catalysts of sulfide mineral weathering and primary producers in benthic marine habitats.

Microbial life in hydrothermal plumes and in the far-field
With the emission of energy-rich, but also contaminant loaded (e.g. heavy metals) fluids into the overlying water column, hydrothermal vents also influence microbial communities in the overlying water column and distant from the vent. Whithin his PhD thesis, Luigi Gallucci studies the ecology of microbial communities along the mixing gradients from fluid discharge to neutrally buoyant plume, with a special focus on the influence of iron on microbial communities in the surrounding of hydrothermal venting and the effect vent microbial communities have on the complexation and distribution of iron into the far-field.
In his thesis, he applies culture-dependent techniques to enrich and study microorganisms with relevance for iron cycling at vents. With various culture-independent, molecular ecology tools, he studies microbial communities in plumes of the Kairei hydrothermal vent field and along the Mid-Atlantic Ridge to assess structure and function of the communities and of individual microbial taxa, and their contribution to trace metal cycling.
Department of Molecular Ecology
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2225 |
|
Phone: |
