- Press Office
- Press releases
- An Ocean of tiny wonders
An Ocean of tiny wonders
Most people associate the production of atmospheric oxygen with terrestrial plants, in particular with the tropical rain forests. However, about half of the atmospheric oxygen is produced by tiny unicellular algae in the oceans. Unlike rain forests, these free-floating algae (termed phytoplankton) live in a more dynamic environment. As a consequence, their productivity is rather cyclic and climaxes during phytoplankton blooms.
Phytoplankton blooms are events of massive algal growth, often occurring in spring when the sun shines longer each day and nutrients are aplenty after lower consumption during winter. The extent of such blooms can be so massive that they are visible in entirety only by satellites, typically as a greenish haze in the ocean. This is usually a good thing, because the entire food web in sun-lit surface waters up to large fish depends on thriving algae. However, occasionally such blooms can consist of harmful algae, as in the red tides that can lead to fish kills and closure of beaches.
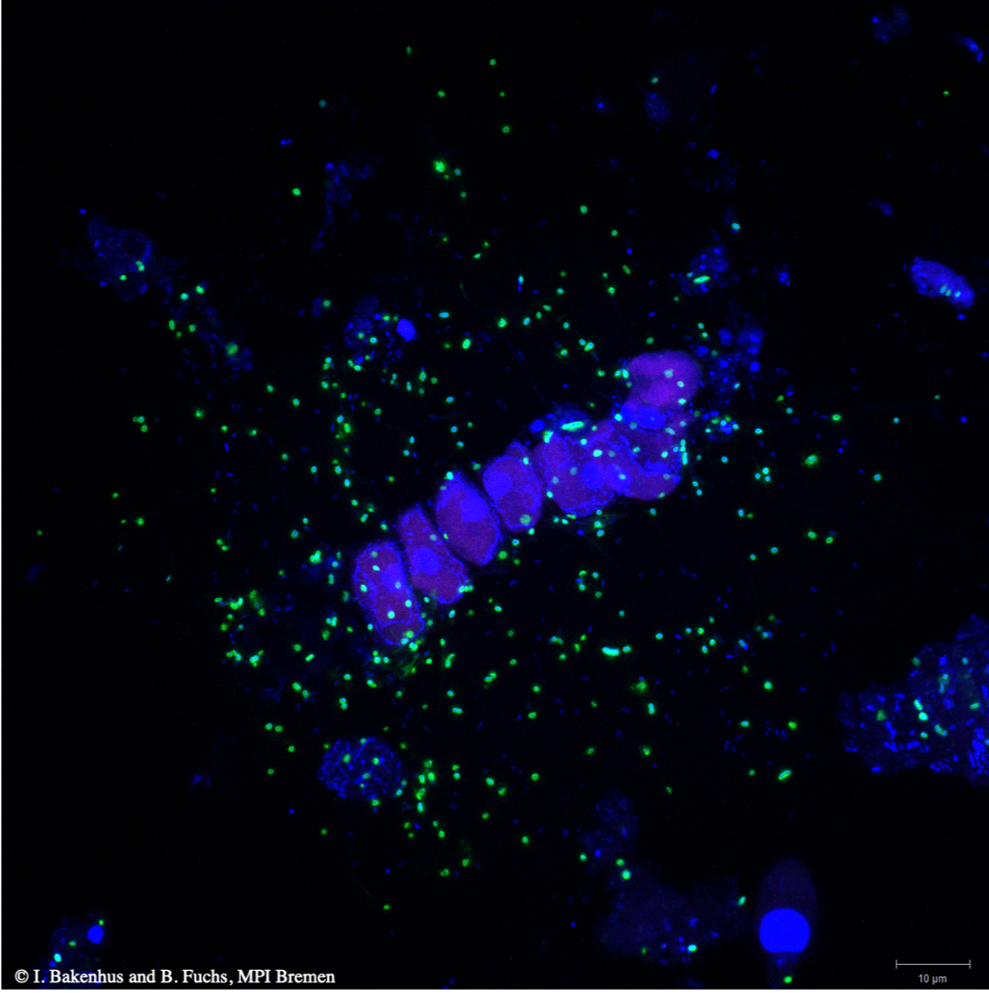
When microalgae bloom, the organic substances they produce feed marine bacteria. This way, blooming microalgae trigger blooms of various clades of bacteria that are either free-living (planktonic), associated with particles, or live in the direct vicinity of the algae (phycosphere). Collectively, these bacteria are able to almost completely decompose algal biomass.
Microalgae may be tiny, but they are plentiful. They produce copious amounts of sugars in form of various polysaccharides, on which many bloom-associated bacteria have specialized. Thus, the bacterial remineralization of these algal polysaccharides plays an eminent role in the global carbon cycle.
Researchers from the Max Planck Institute for Marine Microbiology in Bremen, the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research in Bremerhaven, and the Universities of Bremen and Greifswald have teamed up to explore, which marine bacteria respond to blooming microalgae. The researchers focused in particular on how free-living bacteria decompose the various polysaccharides that microalgae produce, and which polysaccharides they prefer. They now present their results in the journal Microbiome.
As their study site, the researchers chose the station “Kabeltonne” off the island Helgoland in the German Bight. Every spring, planktonic microalgae start blooming at this site. Usually, these algae are for the most part composed of diatoms, one of the most important groups of microalgae that protect themselves with strikingly robust siliceous shells (frustules).
“We regularly took samples from March to May 2020, and analyzed the algal and bacterial compositions”, explains Chandni Sidhu, first author of the study. “As it turned out, the spring bloom in 2020 was dominated by only three diatom species and passed through two very distinct phases. This unusually low algal diversity and the two distinct phases very much simplified the analysis of the responding bacteria. “Usually, we find such simple conditions only in controlled bottle experiments and not in a thriving coastal environment.” The second bloom phase was caused by a phase of steady easterly winds, which carried nutrient rich waters from the rivers Elbe and Weser far into the German Bight.
Sidhu used metagenomics to reconstruct about 250 genomes of bloom-associated free-living bacteria. Subsequently, she used metatranscriptomics to pinpoint the genomes of the 50 most active bacterial species. These species belonged to few bacterial groups that were particularly abundant during the bloom. The latter could be confirmed in the project by direct microscopic cell counting of fluorescently labeled groups of bacteria.
“We could not only follow composition changes in the bacterial community over time, but we could also determine, which genes were switched on and off, including various genes for algal polysaccharide degradation”, says Sidhu. A detailed analysis revealed that many of the active bacteria were decomposing laminarin. Laminarin is a structurally simple beta-glucan that diatoms use to store biochemical energy. Because diatoms are so frequent in the oceans, laminarin is one of the most abundant macromolecules on Earth.
A second class of storage polysaccharides active bacteria consumed were alpha-glucans. Alpha-glucans are used by a wide variety of organisms to store energy, including plants, animals, fungi and bacteria (e.g. as starch or as glycogen). From their data the researchers could show that bacteria were the likely origin of the alpha-glucans. “When bacteria die, their storage glycans are quickly recycled by other bacteria”, explains Sidhu.
The researchers were surprised by the extent to which the planktonic bacteria thrived on readily degradable storage glycans. “We expected to find more active genes for the degradation of other algal polysaccharides, but only few such genes were present”, says Sidhu. “Abundant free-living bacteria clearly preferred to consume the easy stuff, and that is those glycans that are soluble, abundantly present and readily degradable. That leaves many of the more complex and harder to digest algal polysaccharides to the particle-attached bacteria.”
For the first time, the researchers could show, how changes in the availability of laminarin and alpha-glucans during both bloom phases influenced the community composition of bloom-associated planktonic bacteria. Specifically, certain abundant clades, such as Aurantivirga, Cd. Prosiliicoccus and Polaribacter, had a higher preference for dissolved storage glycans, and this influenced their presence during the bloom. Some of these abundant clades also shared similar repertoires of polysaccharide degradation genes, and they obviously competed for the same polysaccharide niche.
Sidhu concludes: “This type of integrated research is only possible when specialists from many different areas work together – biologists from different disciplines, bioinformaticians, chemists and mathematical modelers, to name a few. I am grateful to all the colleagues of our research consortium and associated partners that made this study possible.”
Original publication
Chandni Sidhu, Inga V. Kirstein, Cédric L. Meunier, Johannes Rick, Vera Fofonova, Karen H. Wiltshire, Nicola Steinke, Silvia Vidal‐Melgosa, Jan‐Hendrik Hehemann, Bruno Huettel, Thomas Schweder, Bernhard M. Fuchs, Rudolf I. Amann & Hanno Teeling (2023): Dissolved storage glycans shaped the community composition of abundant bacterioplankton clades during a North Sea spring phytoplankton bloom. Microbiome 11:77 (2023).
Participating institutions
- Max Planck Institute for Marine Microbiology, Bremen, Germany
- Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
- University of Bremen, Germany
- University of Greifswald, Germany
Please direct your queries to:
Group Leader
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2222 |
|
Phone: |

Managing Director
Department of Molecular Ecology
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2221 |
|
Phone: |

Project Leader
Department of Molecular Ecology
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2223 |
|
Phone: |

Head of Press & Communications
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
1345 |
|
Phone: |