- Press Office
- Press releases
- How an industrial microbe converts carbon monoxide into biofuel
How an industrial microbe converts carbon monoxide into biofuel
How do you turn toxic waste into fuel? Ask the microbe! A team of scientists from the Max Planck Institute for Marine Microbiology, together with a colleague from the Max Planck Institute of Molecular Cell Biology and Genetics, experimentally demonstrates the molecular tricks used by the gas-converting microbe Clostridium autoethanogenum to transform industrial waste gases into ethanol — a finding with enormous implications for sustainable fuel and chemical production.
A microbe that serves green technology, but keeps some mysteries
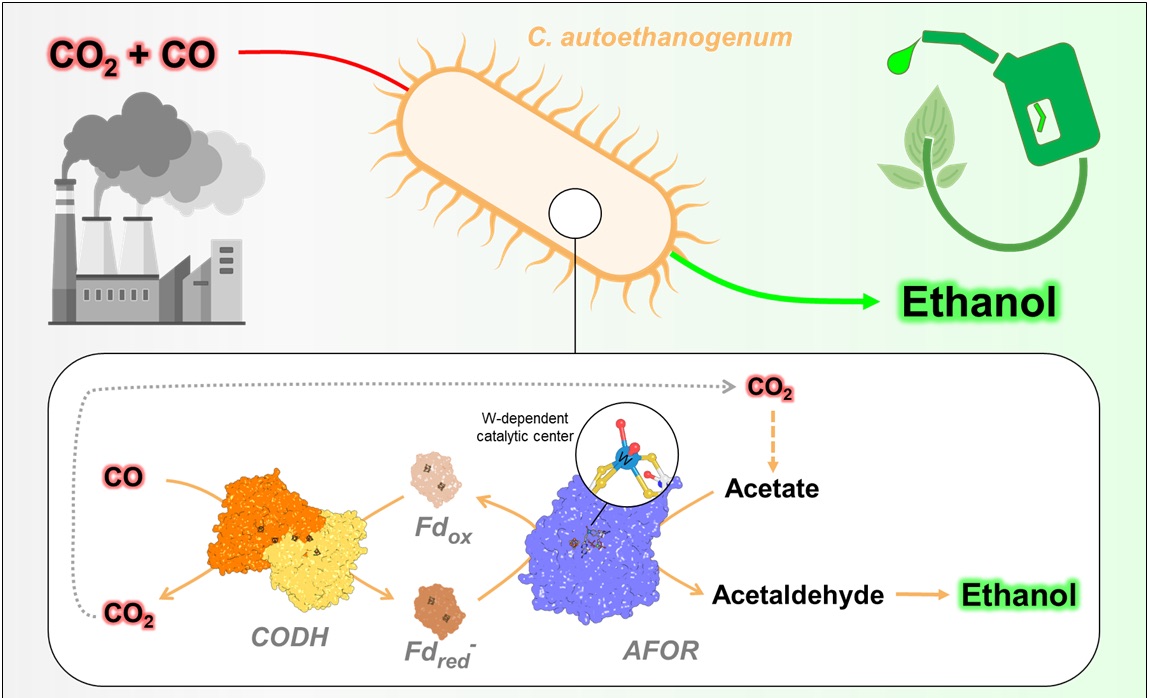
First isolated from rabbit droppings, Clostridium autoethanogenum can thrive on pure carbon monoxide, a deadly gas for most organisms, including human beings. This extraordinary microbe consumes the poison to build cellular materials from the carbon and derives its energy by successive chemical reactions. These reactions can help to produce valuable chemicals and fuels, perfect for driving sustainable biotechnology.
While the organism is currently widely used in industrial plants to produce ethanol, the exact mechanism behind its ethanol production remains unclear. A key step suspected in the reaction was the reduction of acetate to acetaldehyde. However, some scientists did not believe this was possible to carry out for organisms. This study, published in Nature Chemical Biology, now settles this dispute and solves the mystery.
A molecular dissection to understand the reaction of acetate reduction
The enzyme putatively responsible for the challenging chemical reaction is known as the aldehyde:ferredoxin oxidoreductase (AFOR). It contains tungsten, the heaviest atom found in biology. In addition, there is a cluster of iron and sulfur, giving it a pretty dark brown color. Using advanced techniques, the Max Planck scientists purified the enzyme from C. autoethanogenum and determined its atomic structure through X-ray crystallography. With the three-dimensional structure, the scientists depicted the tungsten-containing element and described its surroundings with outstanding precision. However, there was a problem: The enzyme was inactive. “However, with long and hard work we found a way to reactivate it,” explained Olivier Lemaire, who led the research with Tristan Wagner. “Thus, we were able to characterize the enzyme,” says the co-first author of the study, Mélissa Belhamri. “It has a relatively large range of substrates, potentially enabling the production of different alcohols on top of ethanol.”
Enzyme teamwork allows the unfavorable reaction
Next, the scientists needed to explain how AFOR can perform a reaction that is not feasible. “We knew that the enzyme would not reduce acetate easily because of the laws of thermodynamics, so we looked for inspiration in the tricks used by the microbe when growing on carbon monoxide,” said Tristan Wagner. The solution was to mix different enzymes on top of the AFOR. The scientists built an “artificial pathway” in a tube and successfully produced ethanol from acetate, confirming that the full reaction sequence is biologically feasible inside the cell.
Turning waste into useful products
This research provides a long-awaited piece of the metabolic puzzle of ethanol production in this organism, with vast implications. “Discovering the reactivation mechanism of AFOR, its molecular description at the atomic level, and the ethanol production by the artificial pathway, will serve the currently ongoing metabolic engineering of C. autoethanogenum to produce other useful and valuable substances from industrial waste gases,” Belhamri said. The process can also be transferred to other organisms. This will drastically enlarge the potential sources of microbial biofuel production. “This is another step forward in green energy production through gas bioconversion, a clear advance in the circular carbon economy, offering hope for a cleaner and smarter way to recycle carbon”, concluded Lemaire.
Original publication
Olivier N. Lemaire, Mélissa Belhamri, Anna Shevchenko, and Tristan Wagner (2025): Carbon monoxide-driven bioethanol production operates via a tungsten-dependent catalyst. Nature Chemical Biology, online veröffentlicht 29.10.2025 (online vorab als bioRxiv preprint4. September 2024).
Participating institutions
- Max Planck Institute for Marine Microbiology, Celsiusstrasse 1, 28359, Bremen, Germany
- Max Planck Institute of Molecular Cell Biology and Genetics, 01307, Dresden, Germany
- Univ. Grenoble Alpes, CEA, CNRS, Institut de Biologie Structurale, 38000, Grenoble, France
Please direct your queries to:
Head of Group
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany

Head of Press & Communications
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
1345 |
|
Phone: |
