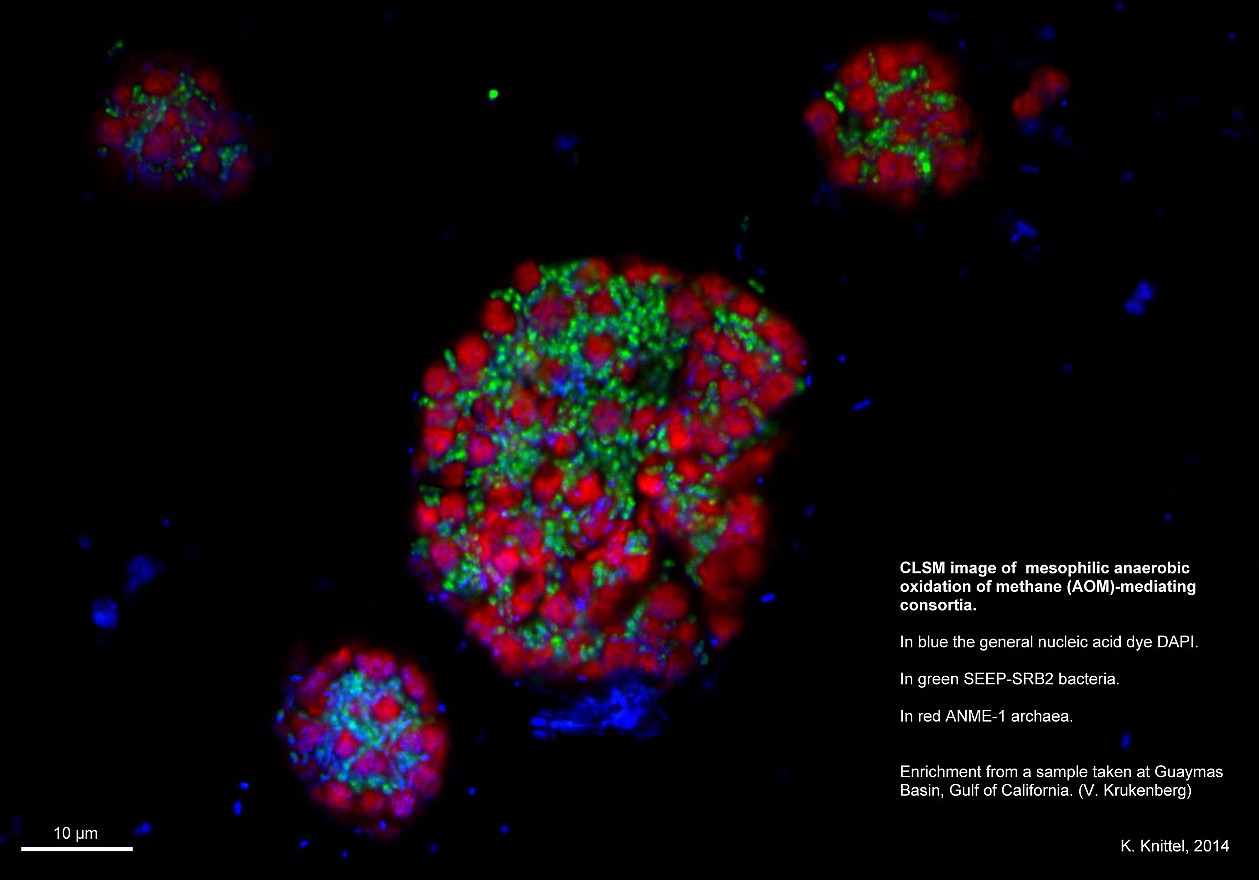
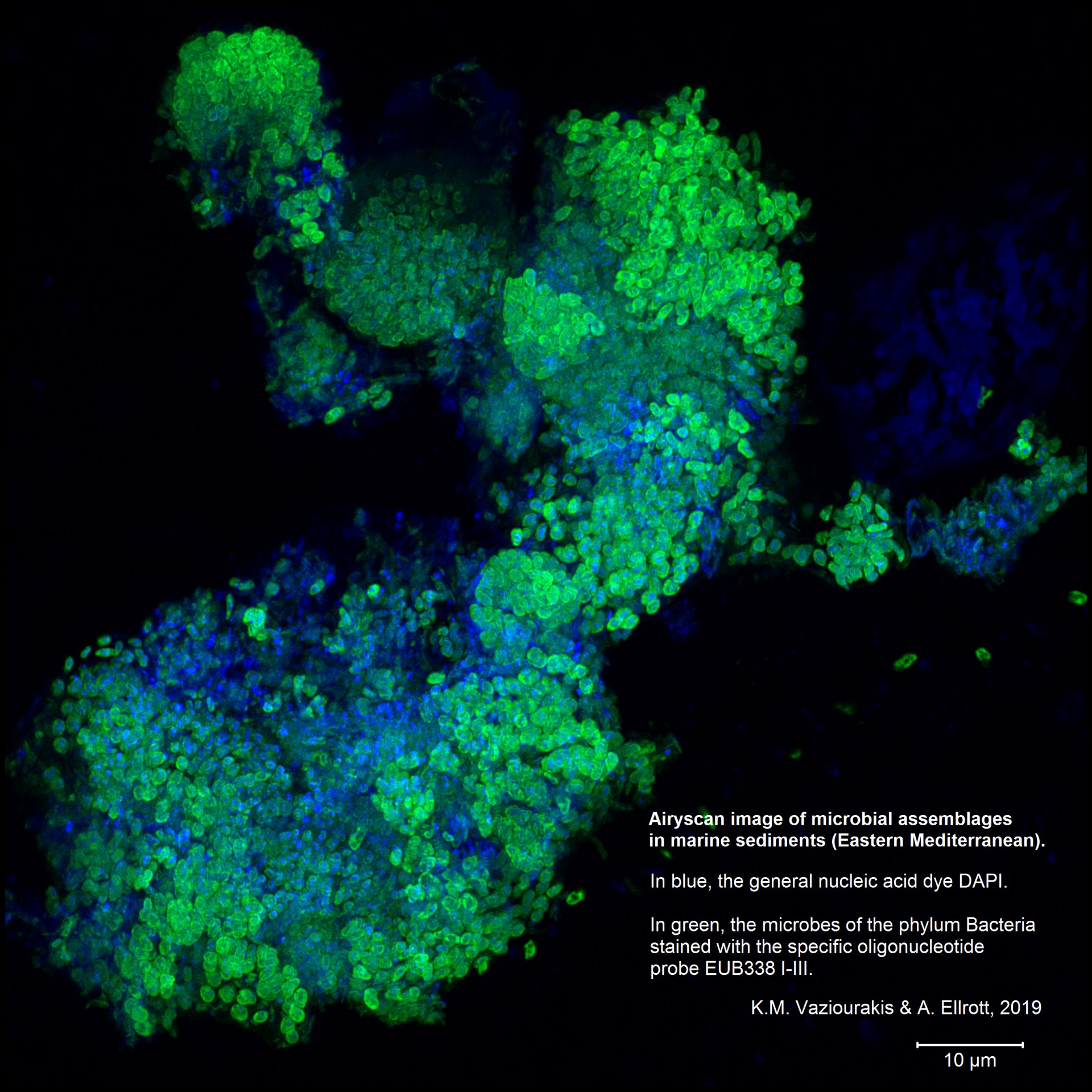
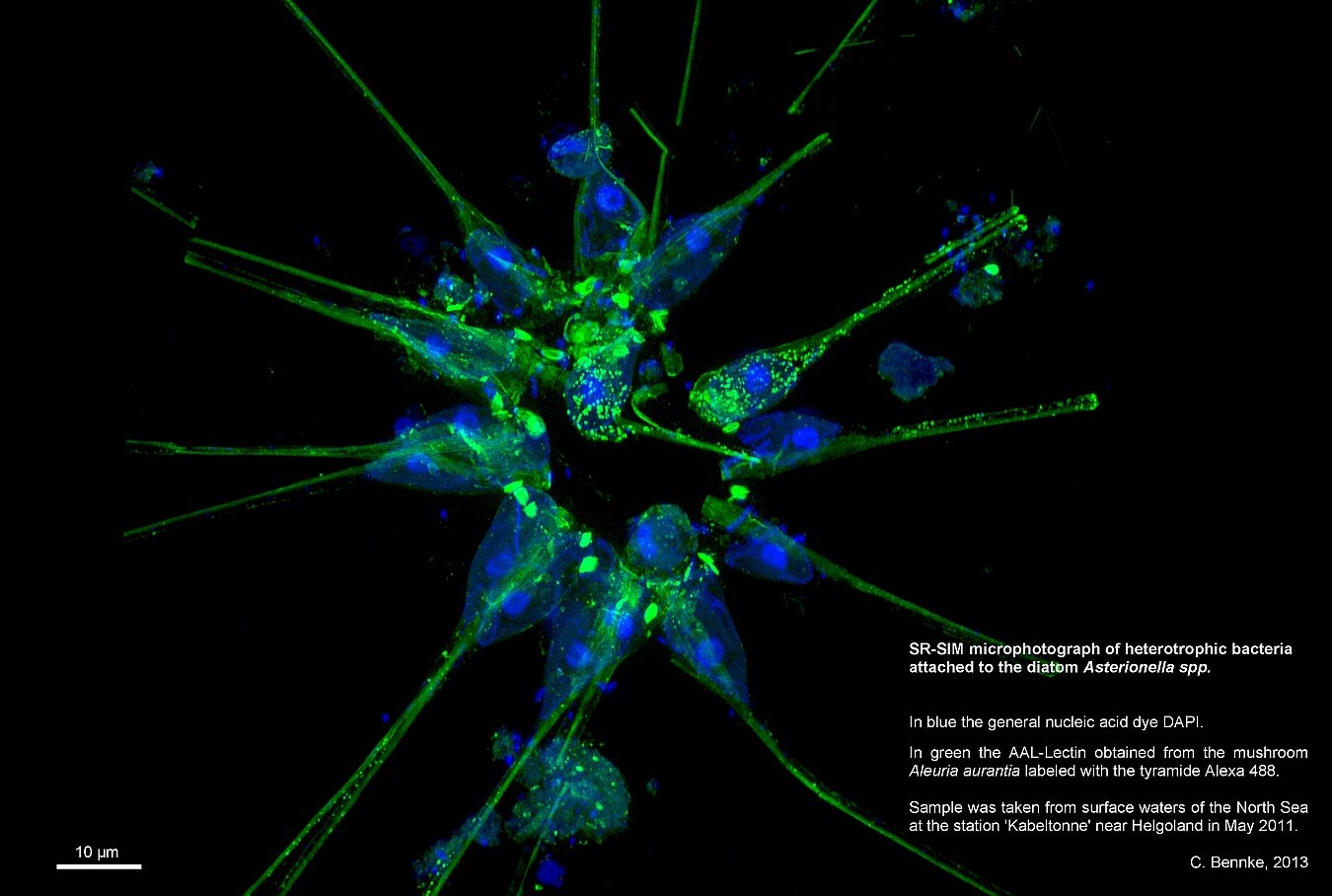
- Research & Instruments
- How we study - Our instruments and methods
- Instruments and Methods
- On Land
- Confocal laser scanning microscope
Confocal laser scanning microscope

What can the confocal laser scanning microscope do?
At the Max Planck Institute for Marine Microbiology, we study mainly micro-organisms from the environment. These are often much smaller than 1 µm and are therefore visible only as a spot under a light microscope. Anything smaller than 200 nm can no longer be seen precisely. The main task of a confocal microscope is to display as accurate an image of minute objects as possible. It allows a more detailed view of the microbes than a standard light microscope.
Why is the confocal laser scanning microscope important?
It is certainly possible to stain the bacteria with fluorescent dyes and visualize them under a fluorescent light microscope. For example, they can be detected and counted in a water sample. However, they are visible only as coloured spots – the individual structures are hardly recognizable. The image is also often blurred because at high magnification, those layers that are not actually in focus blur the image – comparable to a portrait picture in which the background is out of focus.
This is where the confocal laser scanning microscope comes into play. With this device, the respective level or layer relevant to the researcher can be viewed. That means that everything that is not in focus is simply faded out. This creates a sharper image. Structures that were previously blurred can then be recognized more clearly. This, in turn, enables conclusions to be drawn about the structure of a micro-organism.
How does the confocal laser scanning microscope work?
The confocal laser scanning microscope uses a trick with light. With normal light microscopes as well as with the fluorescence microscope, the entire specimen is illuminated. However, things are different with a confocal microscope. Here, a laser is used to scan the area of interest point by point. Software then compiles the information into an image. The fluorescent light is focused by a pinhole diaphragm, which fades out the areas outside the focus. The end result is a sharper image without distracting overlays.
Other methods can also be applied so that more detail can be seen on the images at the same resolution. For this purpose, the confocal microscope has two extensions: an Airyscan detector and the ELYRA high-resolution system with the SR-SIM and PALM or dSTORM techniques.
Airyscan
One problem with a confocal microscope is that there is too little light. An almost closed pinhole allows for a strong focus. However, it is also nearly dark because hardly any light gets through. The strength of the confocal microscope thus becomes a weakness the more the pinhole is closed. Airyscan solves this problem. It consists of several detectors arranged in a circle and resembles a compound eye. Each individual detector has the diameter of an almost closed pinhole and thus “sees” its section as sharply as possible. Because there are 32 such detectors in our version of the Airyscan, each with a specific focal point, there is enough light in total to be able to detect something.
Airyscan can achieve 1.7-fold the resolution of standard microscopy and increases the resolution limit to 140 nm.
ELYRA
The ELYRA system includes two techniques for high-resolution images. The first is called Superresolution Structured Illumination (SR-SIM). It is based on the Moiré effect. This is the effect created when two striped patterns overlap. For example, when someone wears a striped shirt on TV, the audience in front of the screens sees a flicker of the pattern. The flicker is caused by the crossed overlapping of the shirt stripes with the image lines of the camera. This effect can be calculated.
The SR-SIM method works by illuminating the bacteria with a pattern of stripes and then taking a picture. The Moirè effect then occurs where there are natural “stripe” structures in the preparation. This is repeated several times with different positions. Software can then calculate back to the natural structures of the specimen from the Moirè patterns present in the images and thus create a cohesive image. In the best case, it is possible to recognize structures with a resolution of up to 100 nm (i.e. it makes it possible to see twice as well as with a conventional light microscope).
The second technique of the ELYRA extension on the confocal microscope of our Institute is photo-activated localization microscopy (PALM). This is a laborious method because the sample must be specially prepared for this. It is based on fluorescence and takes advantage of the fact that if not all fluorescence molecules light up at the same time, the centre of each light spot corresponds to the position of a single fluorescent molecule. The sample is prepared in such a way that, if possible, no two adjacent molecules light up at the same time. These light points are then measured and displayed in a table. The output of this method is therefore not an image but rather a table with the coordinates of the light points. However, a picture can be calculated from this if necessary. Theoretically, a resolution of up to 20 nm is possible with PALM.
Who uses the confocal laser scanning microscope?
The confocal microscope is operated by the Molecular Ecology Department. However, it is available to all Departments and groups. Use by external parties is possible upon request. For example, within the framework of a collaboration.
Every new user must first receive a briefing. This is given on request by Andreas Ellrott or Katrin Knittel. In addition to the safety aspect, it is essentially intended to help users gain a deeper understanding of the system and the software. In the end, not only beautiful but also scientifically accurate pictures can be taken.
Information on technical details and booking is available at:
READING TIP:
A pupil from Bremen did an internship with us at the Institute for a fortnight and wrote about her experiences with the confocal microscope here: “Olavius in the spotlight” (in german)
Please direct your queries to
Graduate engineer (UAS)
Department of Molecular Ecology
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2204 |
|
Phone: |

Project leader
Department of Molecular Ecology
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2222 |
|
Phone: |