- Research & Instruments
- How we study - Our instruments and methods
- Instruments and Methods
- On Land
- High-performance liquid chromatograph with mass spectrometry (HPLC MS)
High-performance liquid chromatograph with mass spectrometry (HPLC MS)
What is an HPLC-MS?
A high performance liquid chromatograph coupled to a mass spectrometer is one of the most sensitive instruments available for measuring chemical compounds. The analysis combines two separation steps: First, the separation of a mixture of substances on the liquid chromatograph at a pressure of up to 1000 bar. Second, the separation and detection of individual ions in the high vacuum of the mass spectrometer. It is precisely this combination that makes it possible to separate mixtures of substances into their individual components and then precisely measure the molecular masses of these components. An analogy for clarification: Looking for a needle in a haystack is much easier if you can separate the stalks, the leaves, and the needle. And this is exactly what the device does – only on a much smaller scale.
How does an HPLC-MS work?

Two separate devices are connected to each other. The first is an HPLC. Liquid chromatography separates mixtures of substances (e.g. mussel or seaweed extracts) into their individual components. For this purpose, a small amount of diluted extract is applied to an HPLC column, which consists of micrometre-sized beads. The beads are available in different sizes (1.8–5 µm) and materials depending on which components are to be separated. The components of the extract interact with these beads. A solvent mixture (e.g. water and acetonitrile) is then passed over the column in varying proportions. The components of the extract that have the same polarity as the column material remain on the column, while the components that have the same polarity as the solvent are rinsed off the column. At the exit of the column, there is a UV detector, which detects UV-active components of the extract.
The output of the UV detector is connected to the electrospray source (ESI source) of the second device, the mass spectrometer, via a capillary. In this source, the separated components of the extract are ionized. A high voltage is applied to the capillary so that charged droplets are formed at the tip. A neutral carrier gas (e.g. nitrogen) is used to help nebulize the solution and evaporate the solvent. This causes the droplets to disintegrate until only ionized molecules remain. In the analyser part of the mass spectrometer, the ions are separated according to their mass/charge (m/z) ratio. At the end of their trajectory, they hit a detector that registers and counts the ions. This yields a mass spectrum that arranges the signals (peaks) according to their m/z ratio and represents their intensity. ESI ionization in particular often produces multiply charged ions. Here, a doubly charged ion is detected at half its actual mass because it was separated according to its m/z ratio.
HPLC-MS makes it possible to analyse a large number of samples in a short time. This includes both the identification (what is it?) and quantification (how much is there?) of the molecules in the sample. Molecules are usually identified by comparison with databases or reference substances and quantified by adding internal standards and creating calibration curves.
HPLC-MS in teaching
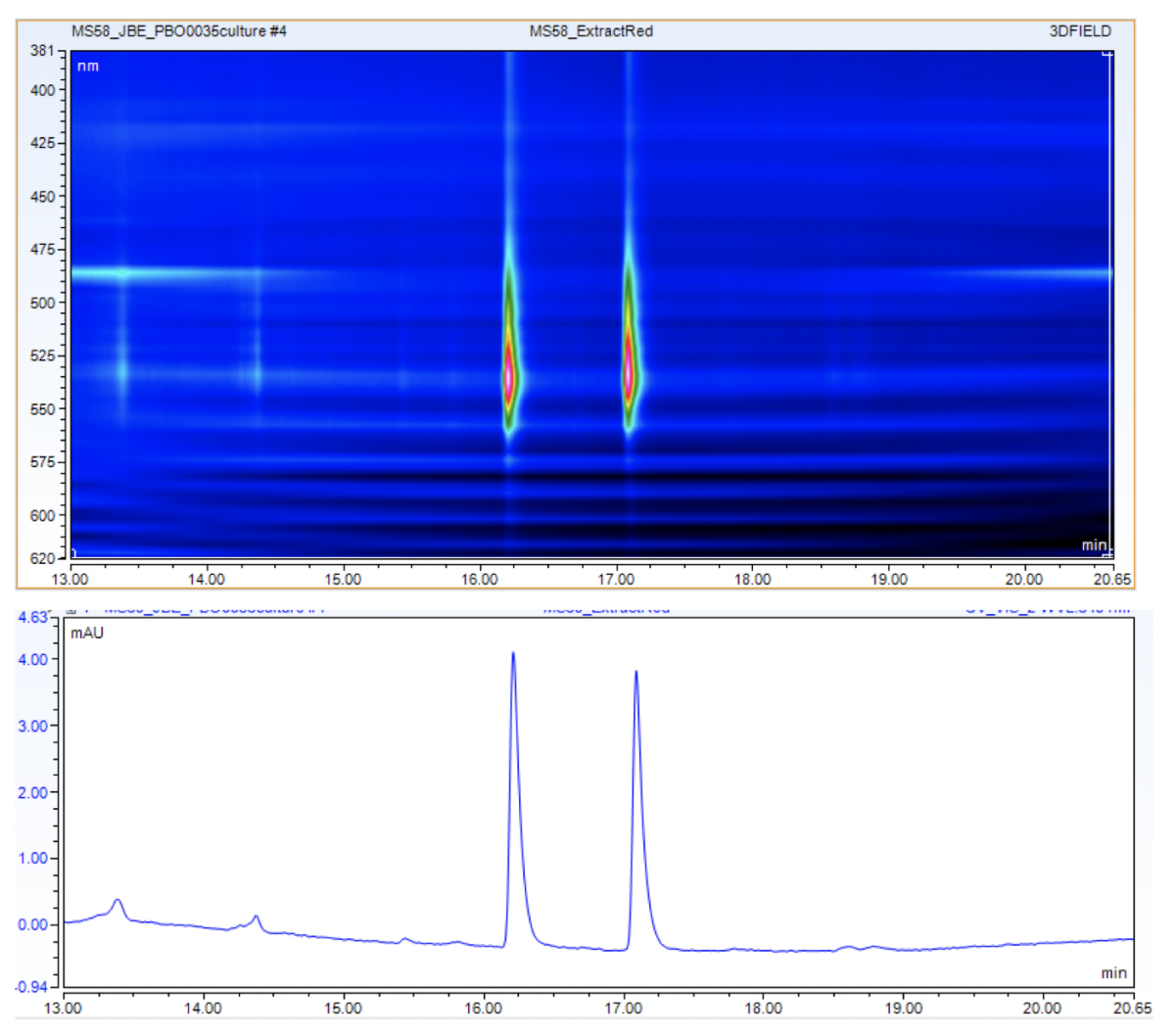
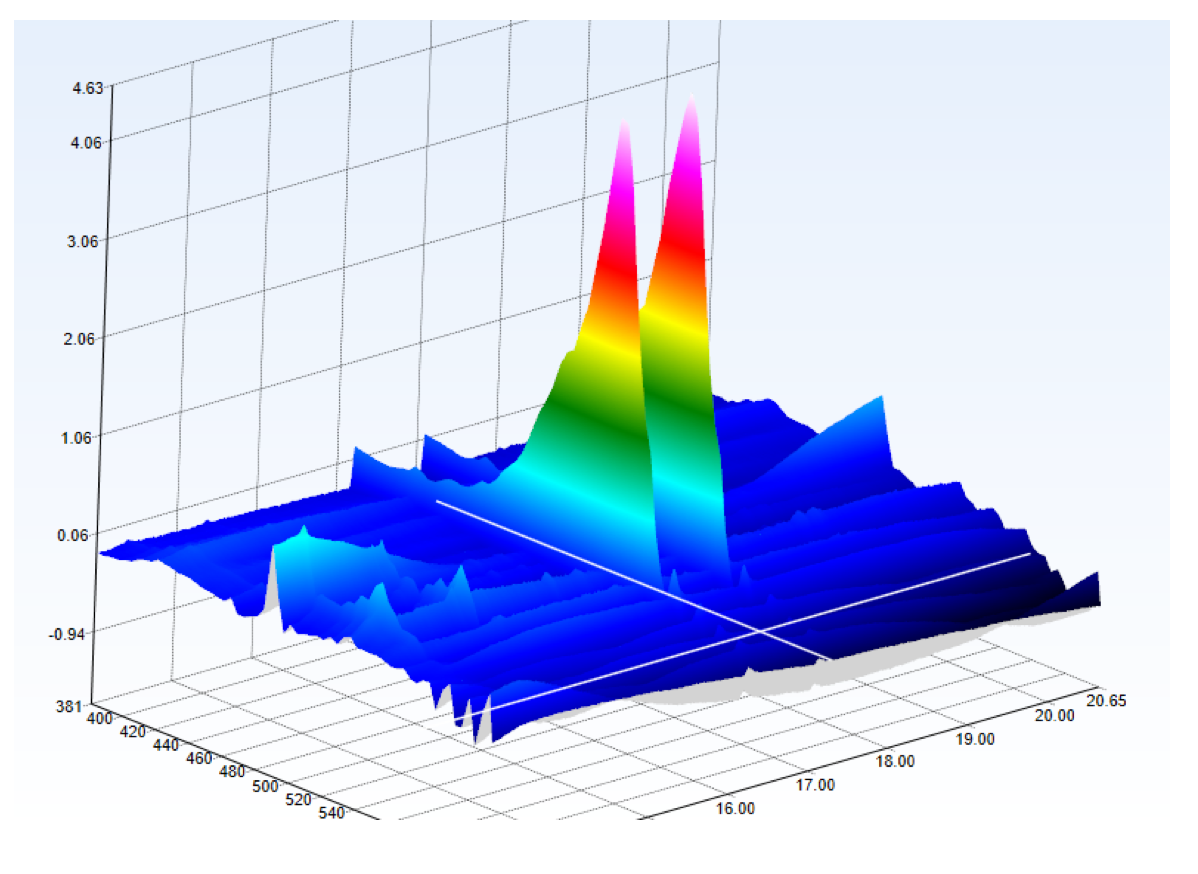
The cultivation and characterization of micro-organisms is an important part of the MarMic Master’s programme, which is taught under the leadership of the Max Planck Institute for Marine Microbiology. A number of marine micro-organisms produce pigments (dyes), which we investigate with the students in the practical component. The students must first isolate a pigment-producing micro-organism and cultivate it in a medium containing glucose and yeast extract (picture left). Methanol, chloroform, and water are then added to extract the pigments from the cells. Because methanol/water and chloroform do not mix, two phases are formed (centre image). The pigments can thus be separated from the cells, and this extract can be analysed with HPLC-MS.
After the colour pigments have been separated on the column, they are detected by the UV detector because they absorb UV radiation. The separated colour pigments are almost simultaneously ionized in the source, separated in the mass spectrometer according to their mass/charge ratio, and detected. The students can then match the molecular structures with the molecular masses detected. In many cases, the exact molecular mass allows the precise determination of the pigment and thus the description of an important property of the respective micro-organism.
Who uses the HPLC-MS?
The device is used mainly by members of the Metabolic Interactions group. But other scientists at the Institute – for example from the Symbiosis Department or the Marine Glycobiology Bridge Group – can also use the instrument for their research.
Contact
Technician
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2506 |
|
Phone: |